XI.
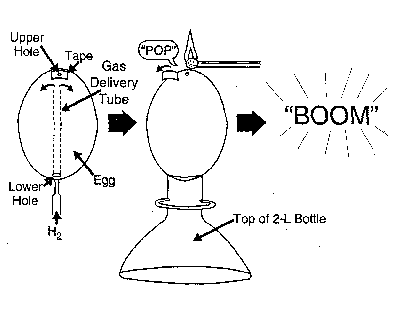
An Egg-Splosive DemonstrationA blown egg is charged with hydrogen gas and then lit at the top. A small "pop" is immediately heard, then 10-15 seconds later, without warning, a crisp, loud report and the entire egg is gone!
Chemical Concepts:
1. Hydrogen reacts with oxygen in a very quick and exothermic manner.
2. Contrary to popular belief, pure hydrogen, by itself, is not explosive. For hydrogen to explode, oxygen must also be present and at a sufficient concentration to react with the hydrogen.
3. Additional energy is often required to get reactant particles to collide hard enough to initiate a reaction; this extra energy is known as the activation energy.
4. As the temperature of a gaseous system increases, the pressure increases as well.
Materials: egg, any size thin, plastic tube (OD <3 mm)
electrician’s tape or putty, 2-L plastic soda bottlesource of hydrogen gas , safety shield

Procedure:
To blow the egg, first wash it thoroughly—there’s no telling where it may have been! Then, with an ice pick or nail, gently tap a small hole (2-3 mm in diam.) in the top (narrower end) of the egg and a slightly larger hole (3-4 mm in diam.) in the bottom. Insert a wire and stir up the yolk. Over a bowl, blow into the small hole to force the egg’s contents out through the larger hole. Then, rinse out the inside several times, and allow the egg to dry overnight at room temperature or for 30-40 minutes in a warm (1000C) oven.
Cover the top hole with a piece of tape or putty and introduce the hydrogen slowly through the bottom hole using a thin delivery tube extended upward almost to the top of the egg. Charge the egg for 30-40 seconds to ensure a thorough flushing out of any air.
Using the top portion of the soda bottle as a Support, stand the egg in an upright position with the taped hole on top. (Remember to have a safety shield between the egg and the audience.) Then, to initiate the combustion reaction, simply remove the tape and hold a burning match briefly to the top hole. Then. stand back! After a small initial "pop" sound, nothing appears to happen. Then, a short while later: "BOOM," and the egg is gone.
Discussion:
The initial pop is caused by a small portion of the hydrogen leaking out, mixing with the air immediately above the hole and forming a minute combustible mixture there. Then, as more hydrogen gas slowly escapes through the top hole, it continues to react with the oxygen in the air and bums with an essentially invisible and silent flame. At the same time, oxygen-containing air is being drawn up into the egg through the bottom hole and mixing with the remaining hydrogen there.
When enough air has entered to form a combustible mixture inside the egg, the flame backfires down through the hole and ignites the mixture. Since the reaction is very exothermic, and since the holes in the egg are not large enough to accommodate the rapid expansion of the gases, the pressure inside the egg increases rapidly and explodes it into several small pieces.
Reaction: 2 H2(g) + 02(g) —~ 2 H20(g) + Q
Tips:
1. The source of hydrogen may be a lecture bottle or simply a reaction between 2 M HC1 and mossy zinc—in a flask with a one-hole stopper and a delivery tube extending directly into the egg.
2. A plastic milk straw or the stem of a thin stem pipet can serve as an adequate delivery tube for the hydrogen. Simply wrap enough electrician’s tape around one end to connect snugly into the stopper or tubing being used.
3. Since the egg-shell pieces are not that substantial, it does not take much to deflect them, and a cut-away 2-L bottle will suffice in place of the explosion shield. Without some type of deflector, however, there is a possibility that a piece of shell might land in a student’s eye. Thus an acceptable alternative to the shield would be to issue the audience individual safety goggles.
4. Colleagues in the "Weird Science" team have repeated this demonstration using an ostrich egg! If this is attempted, be warned: the explosion is considerably more substantial, a regulation safety shield must be used, and the audience should be cautioned to cover their ears.
5. The time-delay between the initial lighting of the egg and the subsequent explosion can vary quite significantly, lasting anywhere from a fraction of a second up to 30 or 40 seconds depending on the size of the egg, the size of the two holes and the extent to which the air inside the egg was effectively flushed out by the hydrogen.
Safety Precautions:
Hydrogen gas is highly flammable; severe fire hazard. This is a good opportunity to train your students to "cup" their ears when they anticipate hearing a loud noise.
This lab is taken from :
Twenty Demonstrations Guaranteed to Knock Your Socks Off!
by Bob Becker 1994